STATES OF MATTER
What are the Three States of Matter?
The three primary states of matter are the solid, liquid, and gaseous states.
All the materials we see in our daily lives (from ice-cream to chairs to water) are made up of matter. Matter can be classified into different states such as solid, liquid and gas on the basis of intermolecular forces and the arrangement of particles. These three forms of matter can be converted from one state of matter to another state by changing certain environmental factors (increasing or decreasing pressure and temperature, for instance). For example, Ice can be converted from a solid into liquid water by increasing the temperature.
The state of the matter is also characterized by the transition of phases. The transition process signals a shift in structure and can be detected by a sudden change in properties. A separate state of affairs may be defined as any set of states that are separated from any other set of states by a phase transition.
Change in state of matter alters the structure of matter and the arrangement of particles. All of this can be observed by noticing the change in properties.
States of Matter
The condition of the matter is one of the distinct forms that the various phases of the matter take. Four states of matter can be found in daily life: solid, liquid, gas, and plasma. Many other states, such as Bose – Einstein condensate and neutron degenerate matter, are considered to occur only in extreme conditions such as ultra-cold or ultra-dense matter. Other states, such as quark – gluon plasmas, are thought to be possible but remain theoretical for the time being.
The states within the device are in a gaseous, liquid or solid state. Solids are distinguished by a tight atomic bond and a high viscosity, resulting in a rigid form. Most solids are crystalline, in as much as they have a three-dimensional periodic atomic structure; certain solids (such as glass) lack this periodic arrangement and are non-crystalline or amorphous.
The particles (ions, atoms or molecules) are tightly packed together in the solid. The forces between the particles are intense in such a way that the particles can not move freely but can only vibrate. As a result, the solid has a stable, definite shape and a certain volume. Solids can only change their shape by force, as if they were broken or cut.
A liquid is an almost incompressible fluid that conforms to the shape of its container but retains a (nearly) constant volume independent of the pressure. Volume is defined if temperature and pressure are constant. As the solid is heated past its melting point, it becomes liquid as the pressure becomes greater than the triple point of the material.
Within a gas, the molecules have enough kinetic energy such that the impact of the intermolecular forces is small (or zero for the ideal gas) and the normal distance between the adjacent molecules is much greater than the molecular size. The gas has no definite shape or volume, but it occupies the entire container in which it is confined.
Three States of Matter with Examples
There are three states of matter and below are the description of various states of matter:
1. Solids
- The Solid state is one of the fundamental states of matter.
- Solids differ from liquids and gases by the characteristic of rigidity.
- The molecules of solids are tightly packed because of strong intermolecular forces; they only oscillate about their mean positions.
- Whereas, liquids and gases possess the property of fluidity and can easily flow.
- Solids can be defined as the state of matter which has definite shape and volume and has a rigid structure.
- Solids possess the least compressibility and thermal expansion.
Example: Iron (Fe)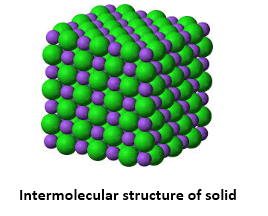
2. Liquids
- The molecules in a liquid are closely packed due to weak intermolecular forces.
- These forces are weaker than solids but stronger than that of gases.
- There is much space in between the molecules of liquids which makes their flowing ability easy.
- Liquids can easily acquire the shape of a vessel, and they have a fixed volume.
- Conversion of solids into liquids takes place when we increase the temperature of solids to a point where solids begin to melt.
- Generally, the density of liquid lies between the density of solids and gases. Compressibility and thermal expansion of liquids are slightly higher than that of solids.
Example: Water (H2O)
3. Gases
- In this state of matter, distances between the molecules are large (intermolecular distance is in the range of
10−7–10−5cm )). - The intermolecular forces experienced between them are negligible.
- Thus, translatory, rotatory and vibratory motions are observed prominently in gases.
- Gases do not have any fixed shape or volume.
- They also possess high compressibility and thermal expansion.
Example: Oxygen (O2)
Plasma
A gas is usually converted to a plasma in one of two ways, e.g., either from a huge voltage difference between two points, or by exposing it to extremely high temperatures. Heating matter to high temperatures causes electrons to leave the atoms, resulting in the presence of free electrons. This creates a so-called partially ionised plasma. At very high temperatures, such as those present in stars, it is assumed that essentially all electrons are "free", and that a very high-energy plasma is essentially bare nuclei swimming in a sea of electrons. This forms the so-called fully ionised plasma.
The plasma state is often misunderstood, and although not freely existing under normal conditions on Earth, it is quite commonly generated by either lightning, electric sparks, fluorescent lights, neon lights or in plasma televisions. The Sun's corona, some types of flame, and stars are all examples of illuminated matter in the plasma state.
- In this state of matter, distances between the molecules are large (intermolecular distance is in the range of


Comments
Post a Comment